Registration/Certification/Approval of Medical Device in India
The Health Ministry of India has published new Medical Device and IVD regulations to enhance the country’ s Drugs and Cosmetics Act for creating effective regulations. The government has notified Medical Devices Rules, 2017 on 31.01.2017 and it will come into force on January 1r 2018. The suggested medical device rules necessary for regulatory approval impact of the Medical device and IVD sector.
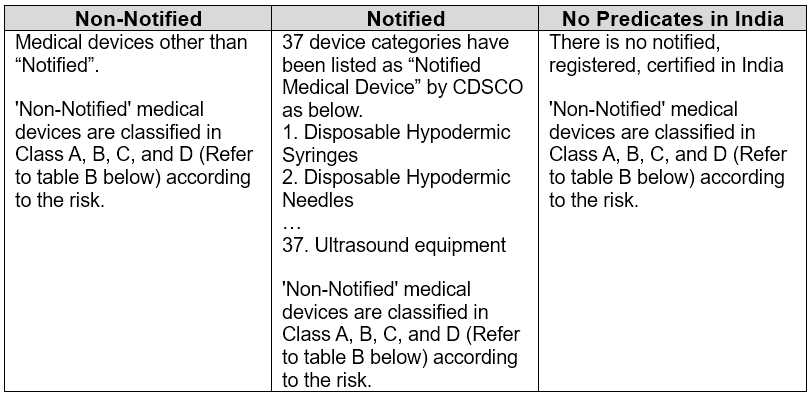
There are three major pathways for the registration/certification/approval of medical devices in India.
- ‘Non-Notified’ Medical Device Registration
- ‘Notified’ Medical Device Certification/Registration
- ‘No Predicates’ Medical Device Approval/Registration
A. Category of Medical Device
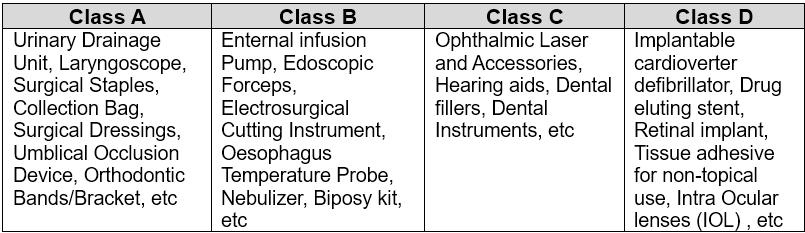
B. Classification of Medical Device
C. Indian Authorized Agent (IAA)
Foreign manufacturers should appoint an Indian Authorized Agent (IAA) to market devices in India. The IAA shall possess active wholesale drug license in the 20B & 21B application forms. Foreign manufacturers may appoint their distributors or importers as the IAA. However, having an independent IAA, with no commercial interest, would provide required flexibility to appoint multiple distributors in India.
D. Compliance Date in Future
- Without the registration number, no medical device can be exported into India after October 1, 2021.
- Class A and Class B devices in “Not Notified” should undergo the “Notified” certification process after October 1, 2022.
- Most Class C and Class D devices are already under ‘Notified’. A few Class C and Class D devices in “Not Notified” should undergo the “Notified” certification process after October 1, 2023.
E. Process Flow

Please contact us for any further question.