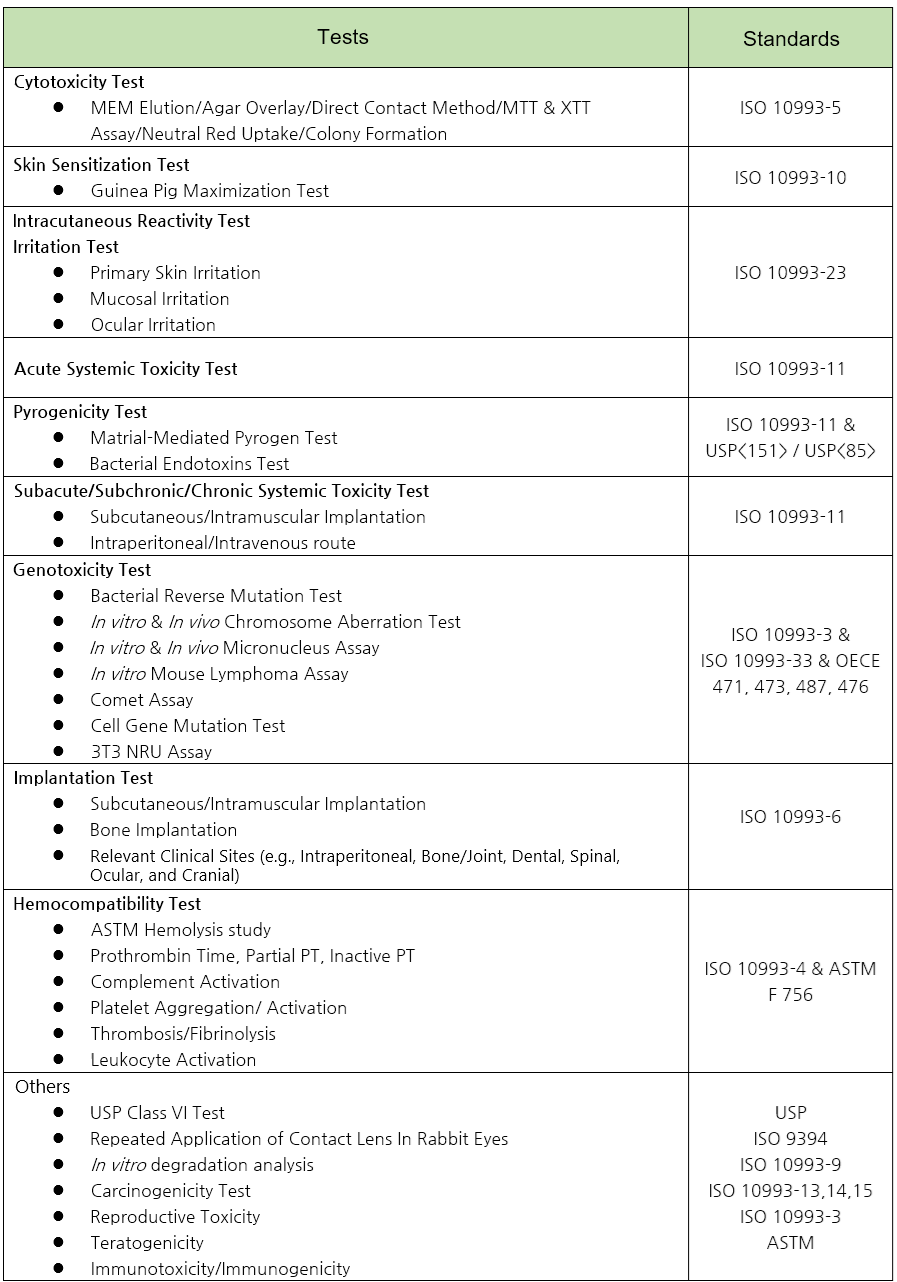
ISO 10993 Biocompatibility (Biological Safety) Testing
Despite the significant costs incurred in conducting many biocompatibility tests, it is not uncommon for these tests to be rejected by regulatory agencies during international approval processes. This often occurs because the tests were not conducted in compliance with ISO 10993 Series standards or because the reports fail to provide sufficient evidence of compliance.
Wise Company aims to address these inefficiencies by collaborating with testing laboratories that have successfully passed FDA inspections. We provide testing services that are faster, more accurate, and cost-competitive compared to domestic alternatives, offering the following advantages:
- ONE. Accredited Institutions: Compliant with ISO 17025, AAALAC, and GLP standards, as well as OECD GLP and FDA GLP (21 CFR Part 58) requirements.
- TWO. Cost and Time Efficiency: Lower testing costs and shorter durations compared to domestic GLP institutions.
- THREE. Expert Support: Test design and constant consultation with experts in FDA/CE/MFDS regulatory approvals.
- FOUR. Customized Reports: Issuance of test reports that meet the requirements of Japan’s Ministry of Health, Labour and Welfare (MHLW), if needed.
- FIVE. Regulatory Submission: Test reports are prepared to comply with and be submitted to regulatory bodies such as the MFDS (Korea), European NBs, the U.S. FDA, and Japan’s MHLW.
- SIX. Comprehensive Testing: Capable of conducting all tests in accordance with the ISO 10993 Series standards.
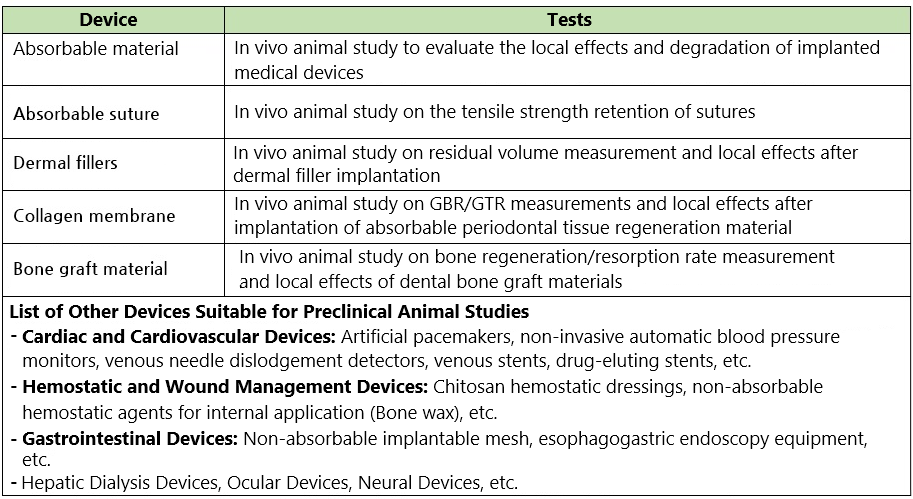
GLP Preclinical Animal Testing
Wise Company collaborates with overseas partner laboratories to provide preclinical animal testing services, offering the following advantages:
- ONE. Accredited Facilities: Partner laboratories are certified under ISO 17025 and OECD GLP and conduct tests in compliance with FDA GLP (21 CFR Part 58) standards.
- TWO. Extensive Experience: Partner laboratories have over 30 years of GLP experience and a track record of submitting more than 300,000 reports for international regulatory approvals.
- THREE. Expert Support: Wise Company provides test design consultation and seamless communication with FDA/CE/MFDS regulatory experts.
Biocompatibility (Biological Safety) Testing for MHLW in Japan
Biocompatibility testing for Japanese regulatory approval must comply with both ISO 10993 requirements and the guidelines of the Japanese Ministry of Health, Labour and Welfare (MHLW). Test reports that do not meet these requirements will be rejected by the MHLW.
The following tests differ from ISO 10993 Series test methods. Upon request, biocompatibility testing that complies with both MHLW and ISO 10993 Series requirements will be conducted. These test reports are duly submitted and utilized for regulatory approval with the Japanese MHLW.
- – Cytotoxicity
- – Sensitization
- – Irritation
- – Material-mediated pyrogenicity
- – Systemic toxicity (acute, subacute, subchronic, chronic)
For detailed guidance, please send the required test items and target regulatory country along with product information (e.g., brochure or user manual) via email.
For inquiries related to biological safety testing, please contact us using the information provided below.